

The process of filtration cannot separate the components in a solution.
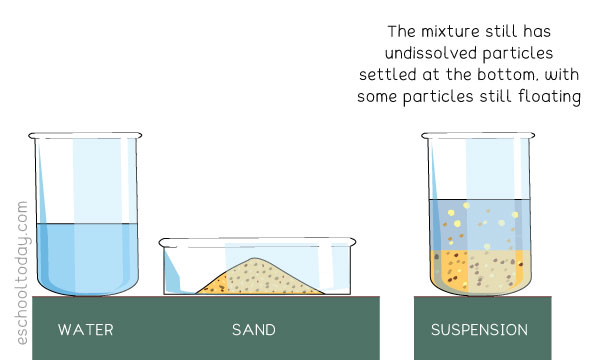
The constituent of a suspension is visible and separated by filtration. The particles in a solution are not visible to the naked eyes. The particles in a suspension are easily visible. 
A Suspension is a heterogeneous mixture.A Solution is a homogenous mixture.Main Differences Between Suspension and Solution The best solutes are sugar, salt, KCl(Potassium Chloride). The solid solution called alloys helps in the manufacturing of cars and other vehicles.

The water solution is helpful in the food, textile, soap, and detergent industry. Turpentine is a solution utilized in the production of paints, dyes, and inks. The property of the solution is useful in industries. There are a few gases, liquids, and even solids that can be solvents. The solution is transparent as the solute is small, and this prevents the scattering of light. The liquid becomes a transparent solution. After the NaCl breaks down, you cannot see the matter. The components are difficult to separate.įor Example, NaCl is a white solid substance. The even distribution of the solute in the solvent makes the mixture homogenous. We cannot distinguish the solvent or the solute even under the microscope. The size of the solute is minuscule <1nm(Nanometre).
Solvent- This is the crucial part of the solution, the water in the saltwater solution. Solute- Is the minor part of the solution, salt in the saltwater solution. A homogenous mixture has a uniform composition which means, the two are not distinguishable. The two components are the solvent and the solute(the substance dissolved in the solvent). The particles are visible and do not dissolve in the liquid. Suspension is heterogeneous means the components that do not mix. The particles of a solution easily pass through the parchment paper. The particles of a suspension do not pass through the parchment paper. It is transparent, the ray of light easily passes through it. It is cloudy, and a ray of light gets scattered. Comparison Table Parameters of Comparison The mixture is transparent, and the dissolving material completely dissolves in the liquid as the size of the solute is less than 1 nm. The components are tough to be separated. The solvent is the dissolving material the solute is the substance that dissolves. It is a homogeneous mixture as the dissolved material uniformly dissolves. A mixture of two substances is a solution.








 0 kommentar(er)
0 kommentar(er)
